![]()
In a single replacement reaction (A + BC -> AC + B), a more chemically active element replaces a less active ion in a solution.
Metallic elements (such as iron, copper, and silver) are insoluble in water, whereas many metallic ions (such as Na+1, Cu+2 and Ca+2), are sometimes soluble in water. In the reaction between copper and silver nitrate solution, copper atoms that makeup the piece of copper are insoluble. The silver ions in the silver(I) nitrate solution are soluble. Again, metallic atoms won't dissolve in water (are insoluble) while many metallic ions will dissolve in water (are soluble).
In the reaction between copper and silver(I) nitrate, a piece of copper is placed in a solution of silver(I) nitrate. Copper is more chemically active than silver. The copper atoms become ions and go into solution while the silver(I) ions become atoms and come out of solution. The nitrate ions remain in solution - they are the spectator ions. In this reaction, silver atoms are produced. They form silver crystals that grow off the surface of the copper.
All chemical reactions involve the losing, gaining or sharing of electrons. In this reaction, a copper atom loses 2 electrons to become a copper ion with a +2 charge. Those electrons go to 2 silver ions (each with a +1 charge) to produce 2 silver atoms. The redox reaction would look like this:
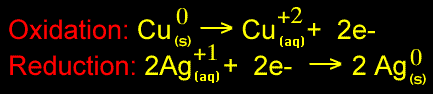
Some of the pictures below were take by Travis Weston, a student in my Honor Physical Science class at SHS - others were taken by me. No special adapter was needed - we just put the lens of our cameras on the eyepiece of the microscope and shot away. The pictures were then brought into FireWorks image editing program where text was added and the pictures were resized. We were both surprised at the clarity of the pictures.
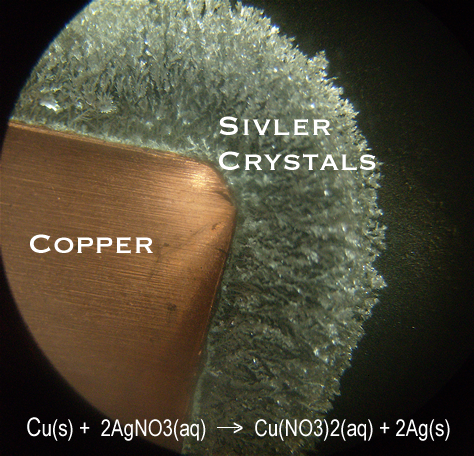
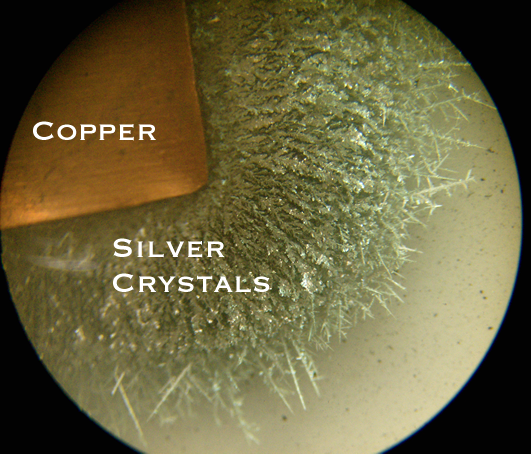
These pictures were taken with two different light sources.

The silver crystals are composed of a silver atoms arranged in a crystalline lattice - a repeating pattern of atoms.
x;j
These pictures have been edited to better show the arrangement of silver crystals.
I shot some other pictures of everyday objects through the microscope. See if you can identify them. The answers are provided below.
Answers listed below
Answers:
1) Jackson's eye on $20 bill
2) Number on a credit card
3) Ball point on a pen
4) T-shirt cloth
5) Computer memory chip
6) Screw
Here are some internet sites that display images taken
through an electron microscope:
Snowflake Crystals
- Images of snowflakes from Cal Tech University.
MicroAngela - Electron
microscope images from the University of Hawaii.
Dennis Kunkel Microscopy - Science
stock microscope photography.
Molecular Expressions - Images from
the FSU Magnet Lab.
Back to Honors Physical Science @ SHS