Synthesis Reactions
In chemical reactions it is important to know the phase (state) of the reactants and products. In many instances if a reactant is not in a certain phase the reaction will not occur. Iron will not rust when placed in solid oxygen. It is therefore important that you identify the phase of the reactants and products in a chemical reaction. To show these phases in the reaction, you will use the subscripts:
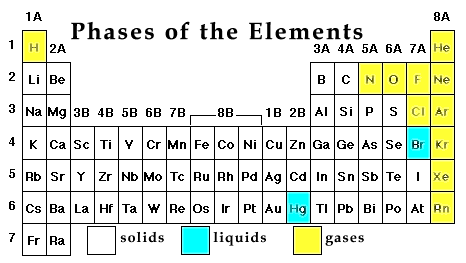
At room temperature most of the elements are solids. All metals are solid except for mercury. All metalloids are solids. Nonmetals such as carbon, phosphorus and sulfur are solids - the rest are gasses. The periodic table below shows the phases of the elements @ room temperature (20ö C, 68ö F).

Synthesis Reactions
Most all metals combine with oxygen. If left out in the air, iron will combine with oxygen to produce iron(III) oxide (rust).The equation for the synthesis of iron and oxygen would read:
* notice that oxygen (as well as hydrogen, nitrogen, fluorine, chlorine and bromine) are diatomic elements - you must place a subscript 2 beneath them when they appear as an element in a reaction.
Most all ionic compounds, such as magnesium oxide are solids at room temperature. Most covalent compounds (such as CO2 and N2O) are gases. A few covalent compounds are liquids (such as H2O).
An electrical current will cause water to decompose into hydrogen and oxygen. This process is called electrolysis.The picture of this shows hydrogen being produced twice as fast as oxygen.

The reactant, water, must be in a liquid phase for the reaction to occur. The reaction will not occur if water is a solid or a gas. The products, hydrogen and oxygen, are both diatomic gases.
Since hydrogen and oxygen gasses are less dense than water, the two gases rise to the surface of the liquid. Arrows pointing up are place in front of the two produces show this.
The equation for the decomposition for water would read:
Single Replacement Reactions
In a single and double replacement reaction, ions must be free to move. When locked in a crystalline lattice, ions are locked in unit cell - their opposite charges (ionic bond) holds them together. Because of this they are not free to move. But when an ionic compound dissolves in water the crystalline lattice breaks apart and individual ions go into solution. In an aqueous solution (water solution) the ions are free to move about.

For a single or double replacement reaction to take place, the ionic compound(s) on the reactant side of the equation must be in solution so a replacement can occur. In a reaction between copper and silver nitrate, the silver nitrate must be in aqueous solution. The reaction would read:
Double Replacement Reactions
In a double replacement reaction, both ionic compounds must be free to move and replace each other. It is for this reason that the reactants are always in aqueous solution. A reaction occurs when one of the products produced in the reaction is not soluble, forms a solid and sinks to the bottom of the test tube. Any solid that forms in a double replacement reaction is called a precipitate.
When a silver nitrate solution is mixed with a sodium chloride solution the silver ions collide with the chloride ions and form a precipitate. The white silver chloride solid then sinks to the bottom of the test tube. An arrow pointing down is placed in front of a precipitate. The reaction would read:
silver nitrate solution plus sodium chloride solution yields silver chloride precipitate + sodium nitrate solution
Review
In synthesis and decomposition reactions:
In single replacement reactions:
In double replacement reactions:
 ---------------------
---------------------