|
2005 - Mr. Gilliland, Honors Physical Science @ Sarasota High |
|
|
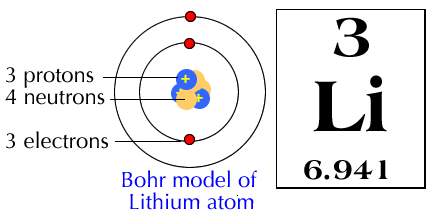
Particle |
Symbol |
Charge |
Location |
Mass |
Importance |
|
Proton |
P+ |
Positive |
Nucleus |
1 AMU |
Identifies the element |
|
Neutron |
No |
Neutral |
Nucleus |
1 AMU |
Isotopes |
|
Electron |
e- |
Negative |
energy levels outside the nucleus |
O AMU |
Determines chemical activity |
You also learned that electrons are found in certain energy levels outside the nucleus. The MAXIMUM number of electrons in an energy level is:
|
|
Maximum number of electrons |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|