It is important to remember that since the number of electrons always equals the number of protons in an atom, the overall charge of an atom is neutral.
But the atoms of most elements are not chemically stable. Here
is the reason why:
The octet rule states that atoms become stable
once they have eight valence electrons.
Atoms engage in chemical reactions in which they lose, gain or share electrons
with atoms of other elements to where they both have a stable electron configuration
(8 valence electrons). The only exception to this rule is H, Li, Beryllium and
Boron. These elements a stable with 2 valence electrons since the first energy
level fills with 2 electrons.
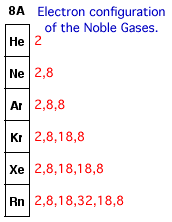
The only elements that have a stable electron configuration are the Noble Gases. You can see that all the Noble Gases have a filled outer energy level.

Atoms of one element will chemically react with an atom of another element so that both have eight valence electrons. Most chemical reaction involves metals losing their valence electrons and nonmetals gaining those electrons to fill their valence energy level. Atoms will become isoelectronic (have the same number of electrons) with the nearest Noble Gas (by atomic number) to become stable. Your teacher will explain this later in greater detail.
Cations & Anions
Metals lose all their valence
electrons to fall back on the filled inner electron energy level.
When metals, like magnesium, lose electrons in a chemical reaction, they become
positively charged ions called cations
(pronounced cat-ions). Magnesium has two valence electrons that it
loses in a chemical reaction. When this occurs, magnesium becomes a cation with
a +2 charge. It becomes charged +2 because it has 12 +protons but only 10 -electrons.
Math will always enable you to determine the charge on an ion (12+) + (10-)
= +2.

When naming cations, just write the name of the metallic element and add ion. As you can see in the above diagram, a magnesium atom becomes a magnesium ion when it loses 2 electrons.
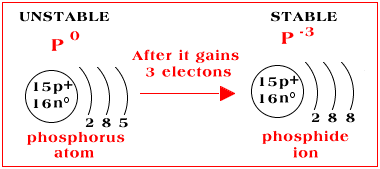
Nonmetals gain valence electrons to complete their valence energy level. When nonmetals, like phosphorus, gain electrons in a chemical reaction they become negatively charged ions called anions (pronouced an-ions). Phosphorus has five valence electrons and will gain 3 more in a chemical reaction. When this occurs phosphorus becomes an anion with a -3 charge. It becomes charged -3 because it has 15 +protons but 18 -electrons. Math will always enable you to determine the charge on an ion (15+) + (18-) = -3.
In an ion the # protons never
equals the # electrons.
Ions always have a + or - charge.
The name of an anion is different from the name of the atom. As you can see a phosphorus atom becomes a phosphide ion. When naming anions, drop the last 2 or 3 letters of the elements name and add the suffix -ide. Here is a list of common anions:
N-3 = nitride
O-2 = oxide
F-1 = fluoride
P-3 = phosphide
S-2 = sulfide
Cl-1 = chloride
Br-1 = bromide
I-1 = iodide
The period table will help you decide what an atom will do in a chemical reaction to become stable. The Alkali Metals, with one valence electron, will lose one e- and become a +1 cation The Halogens, nonmetals with 7 valence electrons, will gain 1 electron and become a -1 anion.

To continue, click on the first member of the family that will gain 2 electrons in a chemical reaction.