

Polyethylene is formed from the monomer ethene (also known as ethylene).

Ethene is an alkene - a hydrocarbon with a double bond between the carbon atoms. Alkenes are unsaturated hydrocarbons which means that one of the bonds between the carbon atoms can break and reform to produce a new substance.
In the polymerization of polyethylene, one of the two bonds between the carbon atoms in the ethene molecule is broken. The bond then forms between the molecules making a long, repeating chain of CH2.

As you can see from the animation above, one of the bonds between carbon breaks and reforms between the molecules. This links the molecules together to produce a long chain that can be made to any length. (The ~ at the end of the polyethylene means that it goes on and on.)
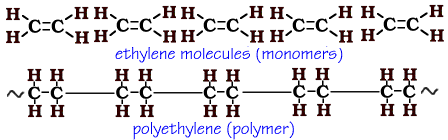
The chemical formula for ethene (ethylene) is C2H4. Monomer formulas are easy - just count the elements. Ethene has 2 carbon and 4 hydrogen. Just remember to place carbon first in an organic formula.
But how are you going to write the formula for the polymer? They consist of millions upon millions of atoms.
Here's how. All polymers have a repeating pattern - one that may be smaller than the monomer. The same structure is repeated over and over again. If you look at polyethylene you see that CH2 is repeated over and over again. When writing a formula for a polymer, you place the smallest repeated formula in parenthesis then the subscript "n" to represent "any number". So the formula for polyethylene is (CH2)n. This formula shows that any number of CH2 linked together make up polyethylene.
Polyethylene is one of the most common of all plastics. It is lightweight, fairly inert (won't react with acids, bases or salts), inexpensive to produce and easy to recycle. It is used to manufacture sandwich and trash bags, milk and soda bottles and other plastic containers.
![]()
![]()